I have started this blog in March 2012, around the same time when pioneering initiatives to treat achondroplasia were just beginning to move from the lab desks to clinical development. Since then, the blog has received close to 500K visits!
However, reaching out to the point where we are now, with one treatment approved and being given to many children around the world, and many others in clinical trials, was not exactly easy.
The cause of achondroplasia, a single point mutation in a key bone growth regulator gene, FGFR3, was identified in 1994 (1) but it was not until 2003 that the first attempts to control the activity of the protein fibroblast growth factor receptor 3 (FGFR3) were published (2).
Almost 20 years after the discovery of FGFR3 as the cause of achondroplasia, the first potential treatment, vosoritide, was brought to the clinic in 2012, in a phase 1 study with healthy volunteers (NCT01590446) and then, in January 2014, to a phase 2 study which enrolled around 30 children with achondroplasia (NCT02055157) (3).
That phase 2 study showed that vosoritide was able to partially restore bone growth velocity (3). In the subsequent larger phase 3 trial (4), after two years of treatment, the effects of vosoritide on bone growth in achondroplasia were considered consistent enough to grant its approval by the main drug development regulatory agencies around the world. Many children with achondroplasia are now being treated with vosoritide and there have been plenty of testimonies published in the social media about kids growing faster then they were before they started treatment.
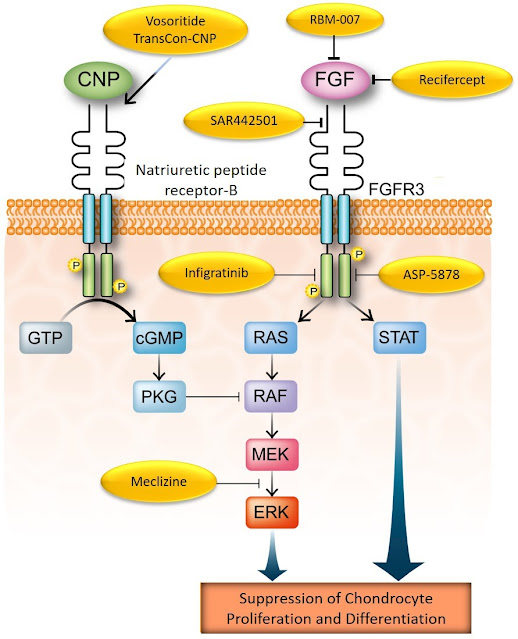
The success of vosoritide attracted other drug developers: at this moment there are at least seven known other potential therapies in development for achondroplasia:
Table 1. List of therapies in development for achondroplasia (not exhaustive).

The above table is an updated version of the one I presented during the ALPE Congress back in October last year (there is another article in the blog about that meeting). Since then, the developers of infigratinib (see here) and TransCon-CNP (see here), have published promising results of their phase 2 studies, which will need to be confirmed in respective phase 3 trials.
However, not all these initiatives have been successful. Recently, Pfizer, which was developing recifercept for achondroplasia, cancelled the program because the drug was not providing any increment on bone growth in children enrolled in their phase 2 study (see here).
Meanwhile, Tyra, a small biotech from California, announced positive pre-clinical results of their TYRA-300 in an animal model of achondroplasia and their intention to take that asset to clinical development (see here).
A search in the literature will also retrieve several other interesting studies evaluating different compounds that seem to have a positive impact on bone growth, directly or indirectly targeting FGFR3.
It's not only about height
I believe that all these work and accomplishments will certainly provide a wide range of benefits for children with achondroplasia. The impaired bone growth that is typical in achondroplasia does not cause only low final stature: there is plenty of evidence that impaired bone growth leads to a series of medical and psychological complications during the life span of affected individuals (5-7). Although it is too early to draw conclusions about any beneficial effects in other aspects linked to impaired bone growth in this skeletal dysplasia, one can estimate that, given these therapies have systemic effect, a decrease in the rate of typical orthopedic complications that affect both children and adults, such as arched legs, spinal stenosis and elbow mobility restrictions, among others, is predictable.
Moreover, recently published studies have also evaluated quality-of-life (QoL) in children and adults with achondroplasia (5-7). In summary, they report that individuals with achondroplasia have lower QoL indexes compared to those of the general population. The impact on QoL has been linked to challenges with daily function, and also physical and mental health. (6,7). There is an expectation that, by improving the length of the long bones, some routine aspects of daily function such as self-hygiene may also improve. With better mobility and function, it is expected that other QoL indexes will improve as well.
It's not only about achondroplasia
One important concept to have in mind about bone growth is that it depends on an intricate system in which many agents work in concert either increasing or reducing the bone growth pace (you can read more about this in other articles of this blog). The fact is that, possibly influenced by all the progress we see for achondroplasia, there has been more research about the mechanism of action of those many bone growth agents in other skeletal dysplasias, too, including FGFR3.
For instance, we now know that in several skeletal dysplasias where FGFR3 is normal, the respective causative mutations seem to lead to FGFR3 axis over-activity, thus contributing to short stature. This has been already identified in RASopathies such as Noonan Syndrome (8), in Cartilage-Hair dysplasia (9), and in diastrophic dysplasia (10).
Furthermore, in skeletal dysplasias where the C-type natriuretic peptide (CNP) axis is not working, drugs that target FGFR3 directly may have an important role in rescuing bone growth. As you may know, CNP regulates FGFR3 activity in normal conditions; if the CNP axis is down, FGFR3 is free to work at will, thus causing severe bone growth impairment. This crosstalk between FGFR3 and CNP was the basis of the development of vosoritide.
Therefore, it is reasonable to think that there is space for the potential use of therapies directed to control FGFR3 activity in other skeletal dysplasias. In fact, there is already one ongoing study with vosoritide in children bearing other skeletal dysplasias (NCT04219007) such as hypochodroplasia (which is also caused by mutations in FGFR3), RASopathies, certain CNP-related dysplasias, SHOX-related dysplasias and ACAN-related dysplasias. Preliminary results from this study have already been released, too (see here).
The future at our door
As typical in the drug development field, there might be other failures ahead. However, drugs like the CNP analogs and those which directly target FGFR3, such as infigratinib, have been showing promising results in the ongoing studies. There will be more options in a few years more.
We are also starting to see research on the gene therapy field. These new generation approaches may, in the future, help to overcome mutations that would otherwise have huge impact on QoL of affected children with many genetic conditions.
From genetic eye disorders or cystic fibrosis, or Duchenne muscular distrophy, to many other conditions which have few to no treatment at all, one can imagine that, when the current technological challenges are resolved, how great it will be to have the possibility to provide a functional gene, for example, to a kid with diastrophic dysplasia.
Based on the current available data, it is possible to envision a time, not far away from now, where children born with many of the genetic disorders such as skeletal dysplasias will be able to enjoy life as any average kid does.
The Treating Achondroplasia blog
Meanwhile, I will keep publishing news and reviews when relevant information about therapies for achondroplasia - and skeletal dysplasias - becomes available. Thank you for your continued interest in this blog!
References
1. Mutations in the gene encoding fibroblast growth factor receptor-3 in achondroplasia. Nature 1994;371(6494):252-4.
2. Fibroblast growth factor receptor-3 as a therapeutic target for achondroplasia--genetic short limbed dwarfism. Curr Drug Targets 2003;4(5):353-65.
C-Type Natriuretic Peptide Analogue Therapy in Children with Achondroplasia. N Engl J Med 2019;381(1):25-35.
4. Once-daily, subcutaneous vosoritide therapy in children with achondroplasia: a randomised, double-blind, phase 3, placebo-controlled, multicentre trial. Lancet. 2020;396(10252):684-92.
5. International Consensus Statement on the diagnosis, multidisciplinary management and lifelong care of individuals with achondroplasia. Nat Rev Endocrinol 2022 Mar;18(3):173-89.
6. Constantinides C, Landis SH, Jarrett J, Quinn J, Ireland PJ. Quality of life, physical functioning, and psychosocial function among patients with achondroplasia : a targeted literature review. Disab Rehabil 2022;44(21):6166-78.
7. Yonko EA, Emanuel JS, Carter EM, Raggio CL. Quality of life in adults with achondroplasia in the United States. Am J Med Genet A 2021;185(3):695-701.8. The ras-GTPase activity of neurofibromin restrains ERK-dependent FGFR signaling during endochondral bone formation. Hum Mol Genet. 2013;22(15):3048-62.
9. Chabronova A, Uncovering pathways regulating chondrogenic differentiation of CHH fibroblasts Non-coding RNA Res 2021;6(4):211-24.