Ten years in a row
The blog Treating achondroplasia is celebrating 10 years online. I have not been posting as often as some years ago but I keep my eyes open to all new relevant information that comes to the field and share them with the interested reader here.
Ten years ago, I started this blog with the main objective of translating the hard jargon that is typical in scientifc publications into a more relatable language that could be accessible to all. The blog has received more than 430K visits since its launch and I hope it has been a helpful source of information for you.
So, to start this new year (I know, it's already February) I want to share with you an updated review about therapies for achondroplasia that I prepared for Fundación Alpe (Gijón, Spain). Alpe is one of the world's strongest advocacy groups for acondroplasia and skeletal dysplasias and you can find the original article translated to Spanish here. This review provides you with high level information about all drugs that are known to be (or could be) in clinical development for the treatment of achondroplasia. You can find more details about them here in the blog: you just need to search for them in the index page.
Nevertheless, the research for therapies does not stop with the drugs listed in Table 1 below. New therapeutic approaches are being explored that we will be reviewing in the next blog's article.
Thank you for your interest in the Treating Achondroplasia blog!
An update of the therapies for achondroplasia
Note: this review has been prepared originally for Fundación Alpe.
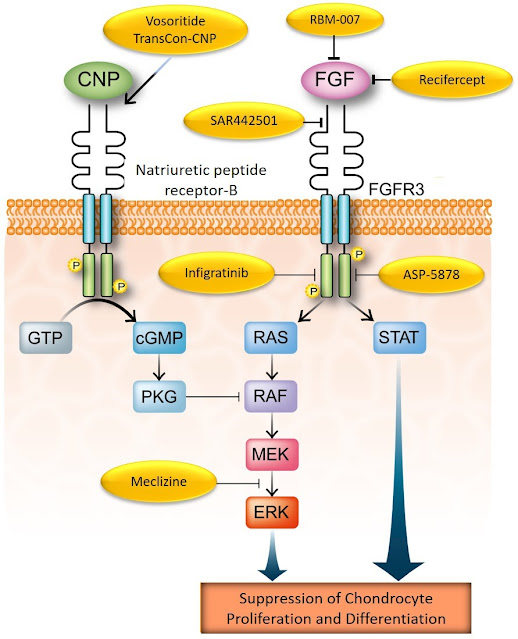
Achondroplasia is the most common form of short-limbed dwarfism. This skeletal dysplasia is caused by a single point mutation in the fibroblast growth factor receptor 3 (FGFR3) gene, which in turn encodes the protein FGFR3, which is located across the cell membrane of the chondrocytes (1). Upon binding of its ligands (the FGFs), it is activated and drives several important cell functions (Figure 1).
FGFR3 has a key role in bone development by regulating the growth plate cartilage function. FGFR3 helps regulating bone growth by, like a brake in a car reducing its speed, counteracting the effect of many other agents which, as the car accelerator, promote bone growth (1). Without FGFR3, bones would elongate without control causing several medical complications (2). In achondroplasia, however, because of the mutation, FGFR3 is working a little too much thereby impairing bone growth (1). More potent mutations in FGFR3 lead to significantly more severe, sometimes lethal forms of skeletal dysplasia.
The consequences of the FGFR3 mutation are well known and go beyond the short stature typical of achondroplasia. Sudden death and neurological problems in early infancy, sleep apnea, recurrent ear infections and multiple orthopedic complications among others throughout life, not to mention significant impact on the quality-of-life, have been already extensively documented in many studies (3,4).
Since the mutation was discovered much has been learned about how it causes achondroplasia. Scientists started thinking and investigating how to control or reduce the activity of FGFR3 to help restoring bone growth. For instance, one of the first objective attempts to target FGFR3 for the treatment of achondroplasia was explored by the group led by Dr. Avner Yayon, who developed an antibody that could block FGFR3 and its activation (5). Unfortunately, FGFR3 works, as we saw above, in the growth plate cartilage, a very special tissue located within the very ends of each of our bones. The growth plate cartilage is a dense tissue that does not receive direct blood supply and these singular features prevent large molecules to transit inside it. As antibodies are very large molecules, in contrast to their vast use in treating many other diseases and in particular cancer, they couldn’t reach their target (FGFR3) in the growth plate, making them inappropriate for the treatment of achondroplasia.
Nevertheless, as scientists were mapping the chemical reactions driven by or affecting FGFR3 (Figure 1), they learned about many other bone growth-promoting agents, too. For instance, they learned that the C-type natriuretic peptide (CNP) pathway plays a key role in bone growth and that it also antagonizes FGFR3 in growth plate chondrocytes. Increasing CNP levels in the growth plate restores, at least partially, bone growth (6,7). With this knowledge in hands, vosoritide has been developed (8), followed by TransCon-CNP (9).
Figure 1. Pharmacological strategies targeting the FGFR3 pathway in the chondrocytes.
Modified from Matsushita M et al. 2013 (10). Reproduced here for educational purposes only.
As FGFR3 may play important roles in some types of cancer, scientists have tried to block it with molecules called tyrosine kinase inhibitors (TKIs) which have the ability to “turn it off” or deactivate it. It was natural to think that a TKI could be explored in achondroplasia. Actually, two of them are in development for achondroplasia (see below) and others might follow the clinical development path (Figure 1).
Scientists also paid attention in how FGFR3 is activated and if it was possible to prevent it. This resulted in the design of molecules like recifercept, a modified FGFR3 molecule that can circulate free, capturing the activators (FGFs) before they reach out to the genuine FGFR3. Using the same strategy, another molecule called aptamer was designed to do the same job, blocking one of the key FGFs before they turn on FGFR3 (Figure 1).
You can find a list of the pharmacological agents being explored for the treatment of achondroplasia on Table 1 and more details about them below.
Table 1. List of current and potential therapies for achondroplasia.
|
Name |
Type |
Developer |
RoA |
Frequency |
Status |
|
Vosoritide |
CNP analog |
Biomarin |
SC |
daily |
Approved |
|
TransCon-CNP |
CNP analog |
Ascendis |
SC |
weekly |
Phase 2 |
|
Infigratinib |
TKI |
QED |
oral |
daily |
Phase 2 |
|
Recifercept |
Ligand trap |
Pfizer |
SC |
daily |
Phase 2 |
|
Meclizine |
Anti-histaminic |
Nagoya University |
oral |
daily |
Phase 1 |
|
RBM-007 |
FGF2 Aptamer |
Ribomic |
SC |
NA |
Phase 1 |
|
Antibody |
Sanofi |
IV(?) |
NA |
Phase 1 |
|
|
ASP-5878 |
TKI |
Astellas |
NA |
NA |
Pre-clinical |
|
ASB-20123 |
CNP analog |
Daichii-Sankio |
NA |
NA |
Pre-clinical |
CNP: C-type natriuretic
peptide. RoA: route of administration. SC: subcutaneous. TKI:
Vosoritide
After long years of clinical development culminating with a successful phase 3 study (11), vosoritide, branded as Voxzogo, has been approved for the treatment of achondroplasia in 2021. In Europe (EMA countries) and Brazil, vosoritide has been approved for children two years of age and older while, in the US, the Food and Drug Administration (FDA) authorized the treatment for children from five years old onward. Other countries will soon follow suit.
Beyond the phase 2 and 3 studies in older children, Biomarin is also testing vosoritide in other three clinical trials, one in infants (NCT03583697), one in children with achondroplasia at higher risk of clinical complications (NCT04554940) and also in a study with other selected forms of genetic growth disorders (NCT04219007).
TransCon-CNP
The main difference between TransCon-CNP and vosoritide is that TransCon-CNP is delivered through a slow-release system allowing a weekly dose with sustained exposure to their analog in contrast with vosoritide's daily dosing. In pre-clinical studies they showed that their CNP analog was superior to vosoritide (9).
Ascendis Pharma is conducting the phase 2 study ACcomplisH with TransCon-CNP. During the JPMorgan 2022 Healthcare Conference in early January (Figure 2), they reported that TransCon-CNP has been well tolerated during the study, with already 65 weeks of drug exposure. They plan to release the data from the phase 2 study in the end of 2022.
Figure 2. ACcomplisH study design (from Ascendis’ JPMorgan 2022 Healthcare Conference presentation).
Infigratinib
Infigratinib is an oral molecule initially developed to treat several types of cancer where FGFRs play an important role for the progression of the disease. It works by blocking the FGFRs' signaling cascades inside the cell (Figure 1). Given that an abnormal, overactive FGFR3 is the cause of achondroplasia, investigators sought to find whether infigratinib could be used to treat this skeletal dysplasia. Preclinical studies demonstrated that it rescued bone growth in a mouse model of achondroplasia, in doses much lower than those used in the first studies in cancer (12,13).
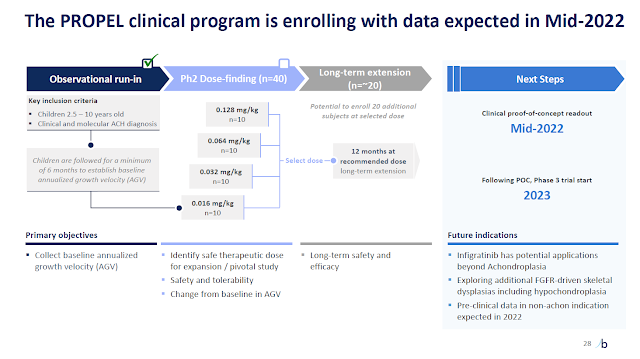
QED, a BridgeBio arm, has been conducting the phase 2 trial called PROPEL and, according to their presentation during the JPMorgan 2022 Healthcare Conference, infigratinib has been showing a safe profile. They estimate to have results from the study by the end of the second quarter this year (Figure 3). Depending on the results they plan to open the phase 3 study right in 2023.
Figure 3. Phase 2 study PROPEL design (from the BridgeBio’s JPMorgan 2022 Healthcare Conference presentation).
Recifercept
Recifercept is a modified, free form of FGFR3 that works as a "ligand trap", capturing FGFR activators (ligands: the FGFs) before they can bind and activate these receptors, including FGFR3. Without the activators FGFR3 would not be as active as expected and this would help restoring bone growth (14,15).
Pfizer has started a phase 2 study with recifercept in the end of 2020 but there have been no significant updates since then.
Meclizine
Drug repurposing is a strategy where investigators try to find new therapeutic indications for old drugs. The concept is that its development for the new purpose should be much less expensive and the final drug cost, if approved, would be surely more affordable than the cost of newly created compounds. Meclizine is an old drug that has been used to treat motion sickness for decades. In an effort to find potential treatments for achondroplasia the Japanese group from University of Nagoya leaded by Dr. Kitoh has found that meclizine was able to inhibit the FGFR3 function and to partially rescue bone growth in their animal model (10,16). They have subsequently conducted a phase 1 study in children with achondroplasia (17). The study showed that meclizine could be suitable for a single daily dose (but that it would need to be further explored in subsequent studies). More recently, they conducted another study to evaluate multiple doses of meclizine for a period of two weeks, but no results have been published yet.
RBM-007
Ribomic has been developing RBM-007, an anti-FGF2 aptamer designed to treat conditions where FGF2 has a relevant role in the mechanism of disease (18). Since FGF2 is considered a key activator (ligand) of FGFR3 and that in achondroplasia FGFR3 is overactive, then if it was less activated by FGF2 perhaps bone growth could be restored.
Ribomic published their pre-clinical studies with RBM-007, which indeed rescued bone growth in a model of achondroplasia (19). They have already started a phase 1 clinical trial to evaluate this aptamer for achondroplasia and are planning to start a phase 2 study in children with achondroplasia during 2022 (Figure 4).
Figure 4. Clinical development plan of RBM-007 for achondroplasia (from Ribomic’s JPMorgan 2022 Healthcare Conference presentation).
SAR442501
Last year, Sanofi announced that SAR442501, an anti-FGFR3 antibody, was transitioned to Phase 1 clinical development. However, no study information could be found on the European Clinical Trial Register, on ClinicalTrials.gov, or the Australian Clinical Trial Register. Apart from being listed in the Sanofi’s pipeline website and in their Feb 2021 financial report (and other reports in their website), no further information about the development of SAR442501 could be found there. Neither pre-clinical data could be found in the Pubmed portal nor through a Google search as of 31-Jan-2022.
Although the antibody strategy is attractive due to its high specificity, the ability of a specific antibody to target FGFR3 in the growth plate in a model of achondroplasia needs to be confirmed in an appropriate model. As mentioned above, the growth plate is a unique body tissue because it lacks direct blood supply. Nutrients and oxygen must traffic through a dense matrix which involves the chondrocytes. In this setting, large molecules such as antibodies might not be able to reach out to chondrocytes to exert their effects (20).
SAR442501 is not the first antibody against FGFR3 to be developed (6). Until recently, B-701 (R3Mab) (21), also known as votafamab, was being developed for cancer and potentially for achondroplasia, but it seems that the research for this indication has been abandoned as there have been no reports about this antibody for this indication for a long time.
ASP-5878
Astellas Pharma has recently published a study where they explored the use of ASP-5878, a TKI similar to infigratinib, in pre-clinical models to treat achondroplasia (22). They found that the drug was able to improve bone growth, however it was less effective compared to a positive control, a CNP analog bearing the same structure of vosoritide.
ASB-20123
Asubio, a Japanese biotech that was recently incorporated by Daichii-Sankio (DS), was developing another CNP analog based on the fusion of the active fragment of CNP and a backbone fragment of the hormone ghrelin to help extending the known short CNP’s half-life. They have published some studies showing that their molecule was able to improve bone growth in pre-clinical models (23) but there has been no news about this compound in the DS website or in the literature lately.
A new era started
With the approval of vosoritide, a new era started. Treating achondroplasia goes far beyond simply improving the final individual height, which nevertheless is an important objective. Although long term data about the effects of vosoritide (and of course the others, too) is not available yet, based on current evidence, there is a fair chance that these therapies may mitigate or prevent several clinical challenges children with achondroplasia face in their daily routine, as mentioned in the beginning of this review.
These children now may have access to a therapy that might help them develop better and have the same opportunities and challenges an average child has while they grow into adulthood. Neither more nor less. I think this is a good perspective.
References
1. Horton WA et al. Achondroplasia. Lancet 2007; 370: 162–72.
2. Toydemir RM et al. A novel mutation in FGFR3 causes camptodactyly, tall stature, and hearing loss (CATSHL) syndrome. Am J Hum Genet 2006;79(5):935-41. Open access.
3. Savarirayan R et al. International Consensus Statement on the diagnosis, multidisciplinary management and lifelong care of individuals with achondroplasia. Nat Rev Endocrinol 2021 Nov 26. Open access.
4. Hoover-Fong J et al. Lifetime impact of achondroplasia: Current evidence and perspectives on the natural history. Bone. 2021 May;146:115872. Open access.
5. Aviezer D et al. Fibroblast growth factor receptor-3 as a therapeutic target for Achondroplasia--genetic short limbed dwarfism. Curr Drug Targets 2003 Jul;4(5):353-65.
6. Golembo M and Yayon A. Method and composition for treatment of skeletal dysplasias. US patent 20040138134. September 2003. Open access.
7. Yasoda A, Nakao K. Translational research of C-type natriuretic peptide (CNP) into skeletal dysplasias. Endocr J 2010;57(8):659-66. Open access.
8. Lorget F et al. Evaluation of the therapeutic potential of a CNP analog in a Fgfr3 mouse model recapitulating achondroplasia. Am J Hum Genet 2012 Dec 7;91(6):1108-14. Open access.
9. Breinholt VM et al. TransCon CNP, a Sustained-Release C-Type Natriuretic Peptide Prodrug, a Potentially Safe and Efficacious New Therapeutic Modality for the Treatment of Comorbidities Associated with Fibroblast Growth Factor Receptor 3-Related Skeletal Dysplasias. J Pharmacol Exp Ther 2019; 370(3): 459-71. Open access.
10. Matsushita M et al. Meclozine facilitates proliferation and differentiation of chondrocytes by attenuating abnormally activated FGFR3 signaling in achondroplasia. PLoS One 2013 Dec 4;8(12): e81569. doi: 10.1371/journal.pone.0081569. Open access.
11. Savarirayan R et al. Once-daily, subcutaneous vosoritide therapy in children with achondroplasia: a randomised, double-blind, phase 3, placebo-controlled, multicentre trial. Lancet 2020; 396 (10257):1070.
12. Komla-Ebri D et al. Tyrosine kinase inhibitor NVP-BGJ398 functionally improves FGFR3-related dwarfism in mouse model. J Clin Invest 2016; 126(5):1871-84. Open access.
13. Demuynck B et al. Support for a new therapeutic approach of using a low-dose FGFR tyrosine kinase inhibitor (infigratinib) for achondroplasia. Approved by but not presented at ENDO 2020 due to COVID-19 pandemics. Accessed on 01-Jan-2022. Open access.
14. Garcia S et al. Postnatal soluble FGFR3 therapy rescues achondroplasia symptoms and restores bone growth in mice. Sci Transl Med 2013; 5 (203):203ra124. Open access after registration.
15. Gonçalves D et al. In vitro and in vivo characterization of Recifercept, a soluble fibroblast growth factor receptor 3, as treatment for achondroplasia. PLoS ONE 2020; 15(12): e0244368. Open access.
16. Matsushita M et al. Meclozine promotes longitudinal skeletal growth in transgenic mice with achondroplasia carrying a gain-of-function mutation in the FGFR3 gene. Endocrinology 2015; 156(2):548-54. Open access.
17. Kitoh H et al. Pharmacokinetics and safety after once and twice a day doses of meclizine hydrochloride administered to children with achondroplasia. PLoS ONE 2020;15(4): e0229639. Open access.
18. Ling Jin et al. Dual Therapeutic Action of a Neutralizing Anti-FGF2 Aptamer in Bone Disease and Bone Cancer Pain. Mol Ther 2016; 24 (11): 1974-1986. Open access.
19. Kimura T et al. An RNA aptamer restores defective bone growth in FGFR3-related skeletal dysplasia in mice. Sci Transl Med 2021 May 5;13(592): eaba4226.
20. Farnum CE et al. In vivo delivery of fluoresceinated dextrans to the murine growth plate: imaging of three vascular routes by multiphoton microscopy. Anat Rec A Discov Mol Cell Evol Biol 2006 Jan;288(1):91-103. Open access.
21. Qing J et al. Antibody-based targeting of FGFR3 in bladder carcinoma and t(4;14)-positive multiple myeloma in mice. J Clin Invest 2009 May;119(5):1216-29. Open access.
22. Ozaki T et al. Evaluation of FGFR inhibitor ASP5878 as a drug candidate for achondroplasia. Sci Rep 2020; 10: 20915. Open access.
23. Morozumi N et al. ASB20123: A novel C-type natriuretic peptide derivative for treatment of growth failure and dwarfism. PLoSONE 2019;14(2): e0212680. Open access.